Name the family to which each organic compound belongs – In the realm of organic chemistry, the classification of compounds into distinct families plays a pivotal role in understanding their structure, properties, and reactivity. This comprehensive guide delves into the diverse world of organic compound families, providing a systematic approach to naming and characterizing these essential building blocks of life.
As we embark on this journey, we will explore the defining characteristics of each family, examining their unique functional groups and the impact they have on the behavior of these compounds. By mastering the art of naming organic compounds, we gain the ability to decipher their complex structures and unravel the intricate tapestry of molecular interactions.
1. Organic Compound Families
Organic compounds are classified into families based on their structural similarities and functional groups. The major families of organic compounds include:
- Alkanes
- Alkenes
- Alkynes
- Alcohols
- Aldehydes
- Ketones
- Carboxylic acids
- Esters
- Amides
Each family has its own characteristic properties, such as solubility, boiling point, and reactivity.
2. Naming Organic Compounds
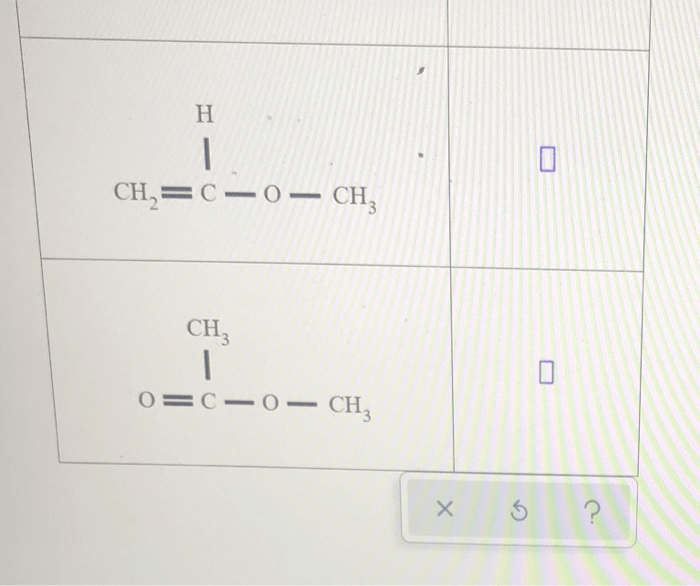
Organic compounds are named according to the International Union of Pure and Applied Chemistry (IUPAC) rules. The name of an organic compound typically consists of:
- A prefix indicating the number of carbon atoms in the parent chain
- A suffix indicating the type of functional group
- Any substituents attached to the parent chain
For example, the compound CH 3CH 2OH is named ethanol because it has two carbon atoms in the parent chain, an alcohol functional group, and no substituents.
3. Functional Groups
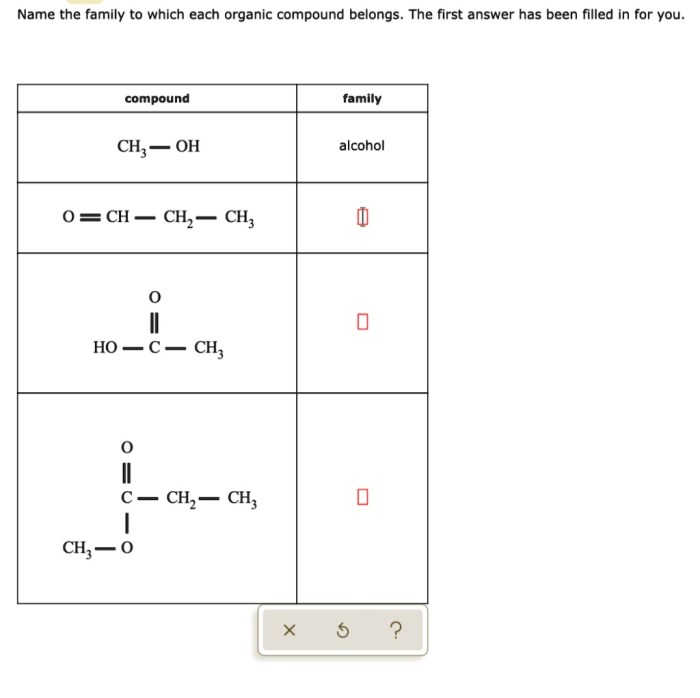
Functional groups are atoms or groups of atoms that give organic compounds their characteristic properties. The most common functional groups include:
- Hydroxyl group (-OH)
- Carbonyl group (C=O)
- Carboxyl group (-COOH)
- Amino group (-NH 2)
- Alkyl halide group (-X, where X is a halogen)
Functional groups determine the reactivity and solubility of organic compounds.
4. Table of Organic Compound Families
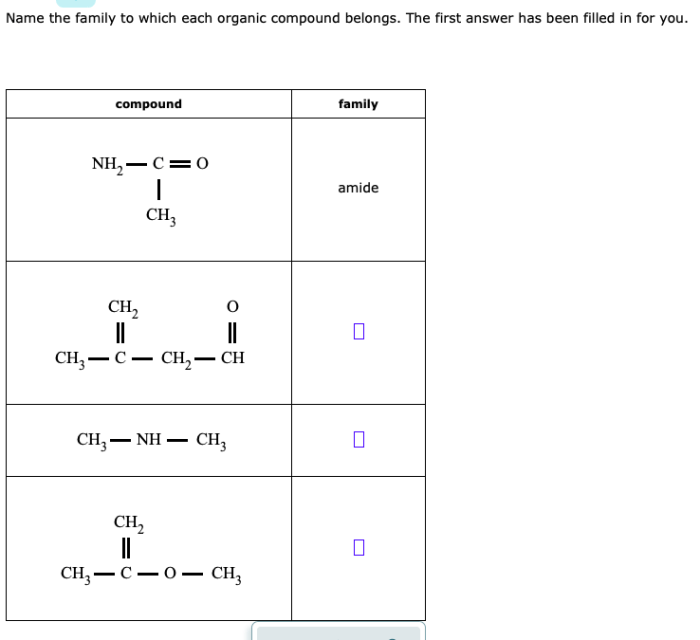
| Family | Examples | General Properties | Functional Groups |
|---|---|---|---|
| Alkanes | Methane, ethane, propane | Saturated hydrocarbons, nonpolar | None |
| Alkenes | Ethylene, propylene, butylene | Unsaturated hydrocarbons, contain one or more double bonds | C=C |
| Alkynes | Acetylene, propyne, butyne | Unsaturated hydrocarbons, contain one or more triple bonds | C≡C |
| Alcohols | Methanol, ethanol, propanol | Contain a hydroxyl group (-OH) | -OH |
| Aldehydes | Formaldehyde, acetaldehyde, propionaldehyde | Contain a carbonyl group (C=O) | C=O |
| Ketones | Acetone, butanone, pentanone | Contain a carbonyl group (C=O) | C=O |
| Carboxylic acids | Formic acid, acetic acid, propionic acid | Contain a carboxyl group (-COOH) | -COOH |
| Esters | Methyl acetate, ethyl acetate, propyl acetate | Contain an ester group (-COO-) | -COO- |
| Amides | Acetamide, formamide, propionamide | Contain an amide group (-CONH2) | -CONH2 |
FAQ Explained: Name The Family To Which Each Organic Compound Belongs
What are the main families of organic compounds?
The primary families of organic compounds include alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and esters.
How are organic compounds named?
Organic compounds are named according to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature system, which assigns a unique name based on the compound’s structure and functional groups.
What is the role of functional groups in organic compounds?
Functional groups are specific atoms or groups of atoms that impart characteristic chemical properties to organic compounds. They determine the reactivity and behavior of the molecule in various chemical reactions.